How to Find the Activation Energy
ΔG ΔH T ΔS. Herein how do you find the activation energy for the reverse reaction.
The Arrhenius Equation Activation Energy And Catalysis Explained Pt 8 Youtube
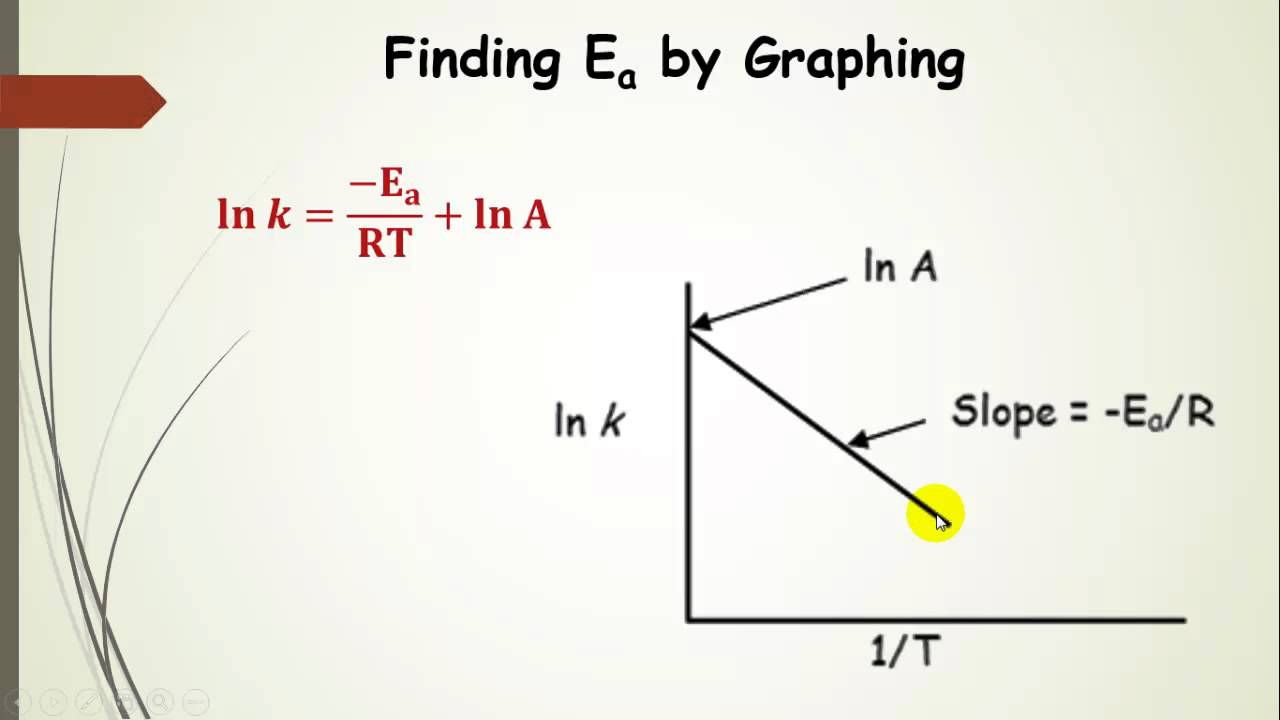
Another way to calculate the activation energy of a reaction is to graph ln k the rate constant versus 1T the inverse of the temperature in Kelvin.

. The activation energy for this reaction is 459 x 104 Jmol or 459 kJmol. Or an intro to activation energy seehttpsyoutubehgQDMmzSOu4To solve for activation energy graphically seehttpsyoutubedrnYpMng90oIn this video we. The plot will form a straight line expressed by the equation.
For example the Activation Energy for the forward reaction AB -- C D is 60 kJ and the Activation Energy for the reverse reaction C D -- A B is 80 kJ. So now we can use it to calculate the Activation Energy by graphing lnk versus 1T. Ea activation energy of the reaction.
More specifically we can write the Gibbs free energy of activation in terms of enthalpy and entropy of activation. The activation energy is equal to the difference between the threshold energy needed for the reaction and the average kinetic energy of all the reacting molecules. Furthermore how do you find the reverse reaction.
Enter the temperature frequency factor rate constant in the input field. The activation energy for the backward reaction is equal to the sum of the activation energy of the forward reaction and the enthalpy change of the reaction. Thus the activation energy of the backward reaction is 301040kJmol1.
Now click the button Calculate Activation Energy to get the result. They use thetha or Tar. As per your value the.
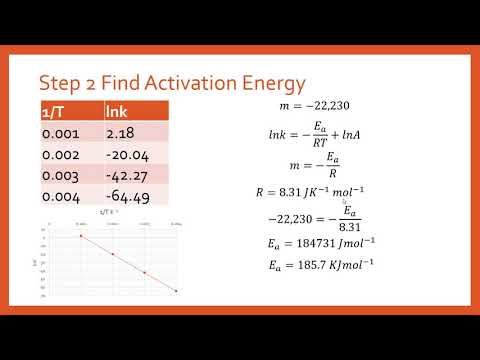
In some cases authors do not use this form and they use the form just like two other form see ar-2 and ar-3. How to find activation energi got slope 0041 conductivity vs temp. One can also derive the activation energy formula in an algebraic manner.
A slight rearrangement of this equation then gives us a straight line plot y mx b for ln k versus where the slope is. Substracting equation 4 from equation 3 results in. The procedure to use the activation energy calculator is as follows.
Shud i multiply it by kboltzmann const or i shud use stantdard formula. Density 500 Celsius 262 grcm 3. For instance three kinds are attachedPlease consider the first attachment.
NOW Activation Energy. Then for a unimolecular one-step reaction the approximate relationships Ea ΔH RT and A kBTh exp1 ΔSR hold. What is backward activation energy.
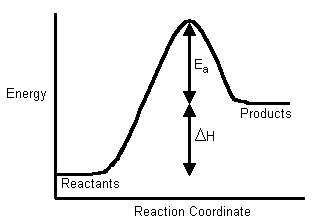
The Activated Complex is an unstable intermediate product that is formed during the reaction. Now I need to calculate Er from thethaTar. Because the reverse reactions activation energy is the activation energy of the forward reaction plus ΔH of the reaction.
The activation energy can also be found algebraically by substituting two rate constants k 1 k 2 and the two corresponding reaction temperatures T 1 T 2 into the Arrhenius Equation 2. We start by taking the natural. T1T2 Absolute Temperature in Kelvin.
E a the activation energy of the reaction in Jmol. K1k2 the reaction rate constant at T1 and T2. M - E a R.
The question is like this. The value of the slope m is equal to -EaR where R is a constant equal to 8314 Jmol-K. In this equation k is the rate constant for the reaction Z is a proportionality constant that varies from one reaction to another E a is the activation energy for the reaction R is the ideal gas constant in joules per mole kelvin and T is the temperature in kelvin.
Activation energy slope1000kbe here kb is boltzmann constant 138010-23 kgm2Ks and e is charge of the electron 1610-19. Now one use it to calculate the Activation Energy by making use of the graphing ink versus 1T. Given two rate constants at two temperatures you can calculate the activation energy of the reactionIn the first 4m30s I use the slope formula y2-y1 x2.
Once the reaction has obtained this amount of energy it must continue on. Ln50 30e-E a 8314679 E a 11500 Jmol. Furthermore ere one undertakes the substitution of two rate constants A1 A2 and the corresponding two temperatures T1T2 into the Arrhenius equation.
We can graphically determine the activation energy by manipulating the Arrhenius equation to put it into the form of a straight line. In that wat is the value of A Pre- exp factor. There is an activation energy Er.
Calculate the activation energy needed to create a single vacancy in aluminium given. Ie E a Threshold energy E Threshold - Average kinetic energy of the reacting molecules E. 11500 Jmol 23 kJmol X 1000 34500 Jmol.
R ideal gas constant83145 JKmol. How do you find activation energy from free energy. Taking the natural logarithm of both sides gives us.
The Arrhenius equation can be used to determine the activation energy for a reaction. Formula to calculate activation energy. T 1 and T 2 absolute temperatures Kelvin k 1 and k 2 the reaction rate constants at T 1 and T 2.
When the lnk rate constant is plotted versus the inverse of the temperature kelvin the slope is a straight line. If we know the rate constant k1 and k2 at T1 and T2 the activation energy formula is. R the ideal gas constant 83145 JKmol.
The activation energy can be determined by finding the rateconstant of a reaction at several different temperatures. Finally the activation energy required for the atoms or molecules will be displayed in the output field. GuysIve seen the Modified Arrhenius equation in several forms.

Arrhenius Equation Activation Energy And Rate Constant K Explained Youtube
How To Use An Arrhenius Plot To Calculate Activation Energy And Intercept Youtube
Comments
Post a Comment